Document Management Tool
— Project scope
Redesign
Overhaul
— Role
Research
Wireframes
Prototypes
UI/UX
— Duration
3 months contract
- The Challenge
- • The workflow wizard is not user friendly & requires heavy training.
- • UI is complicated & results in human errors that requires customer support.
- • It is not designed to scale and adding the features users were requesting would add complexity.
Key Goals:
- • Improve usability by introducing a user friendly solution
- • Reduce human error
- • Design for scale
The approach
- Summary:
The opportunity to re-imagine the feature lend for a design overhaul. Since a system like this is difficult to compare to our competitors I approached it by thinking outside the box and looking and other management tools, such as box, dropbox. The end design concept that was shared with users was a combination of a Box/Drop Box concept and shopping cart.
Research:
- • Internal research and audit and interviews
- • Competitive research and industry best practice
- • Wireframes and prototyping
- • User validation sessions 6 with with 25-30 users
Design:
The first concepts were shared as wireframes. We wanted the feedback sessions to focus on the interaction and not the design.The last few feedback sessions were clickable prototypes that resembled an e-commerce shopping experience
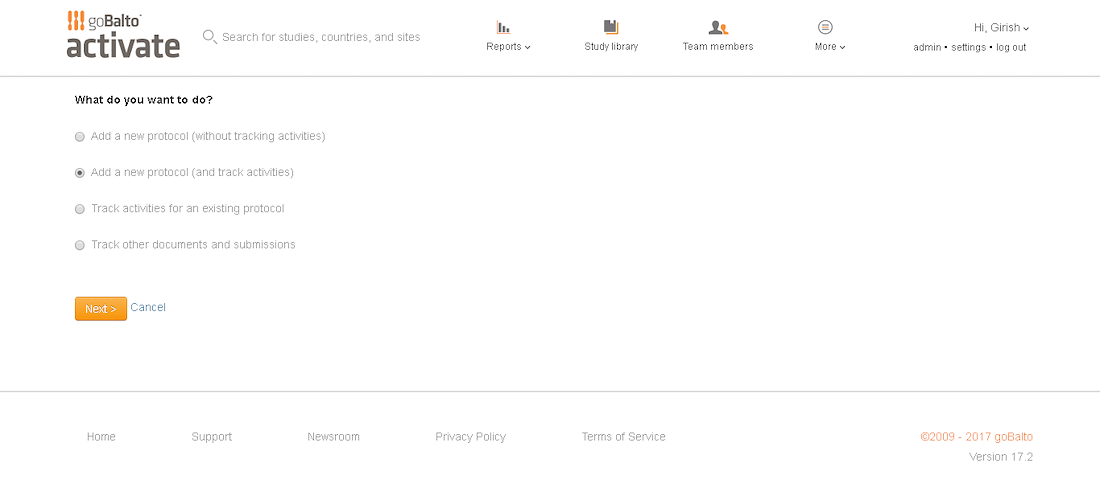
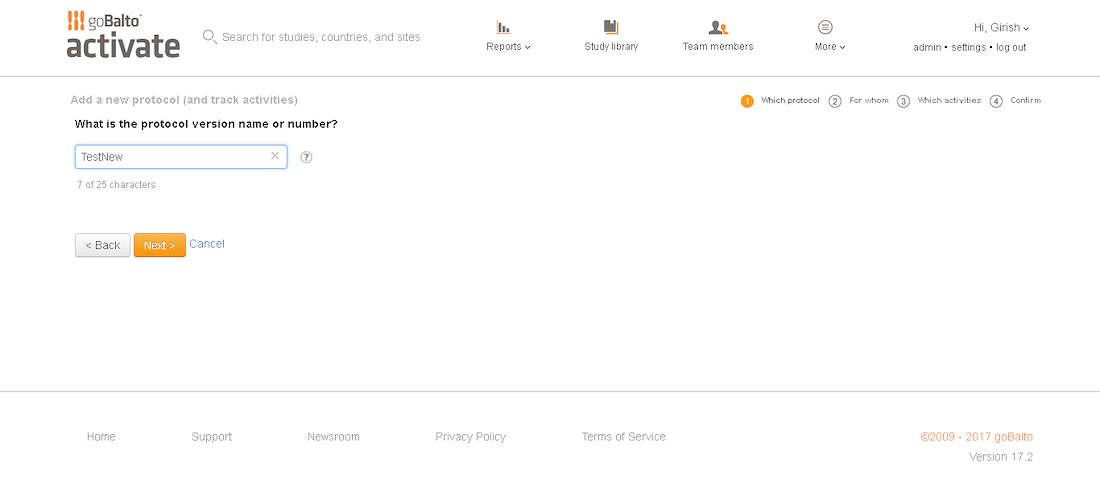
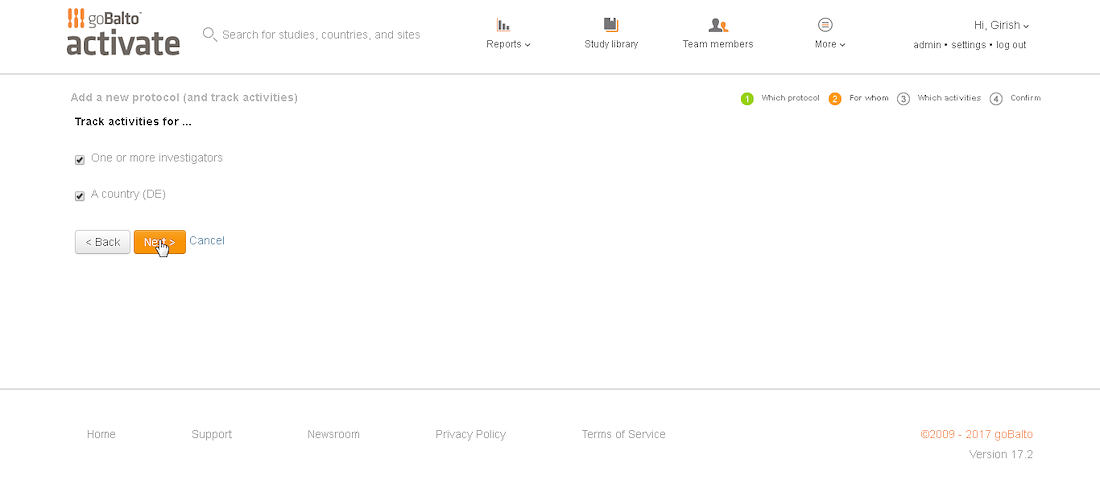
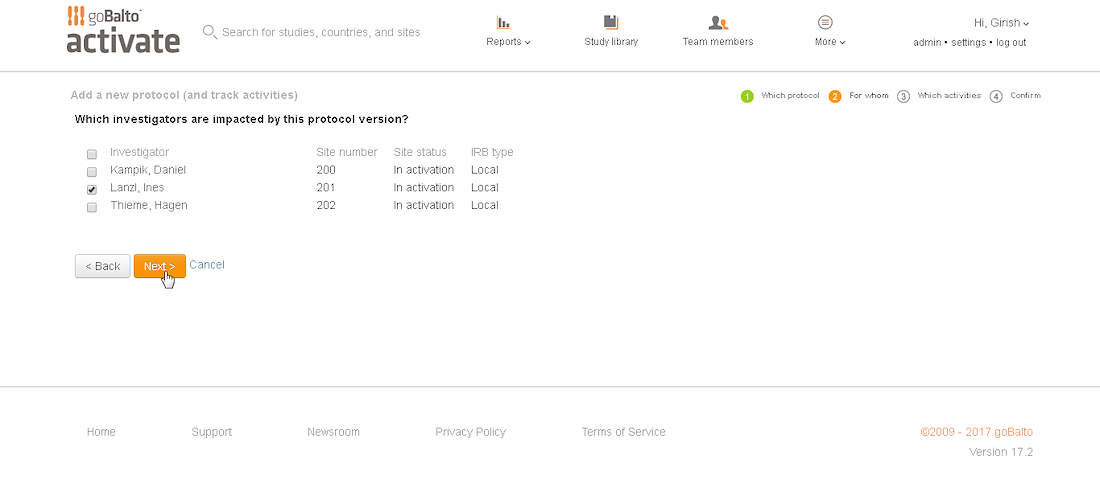
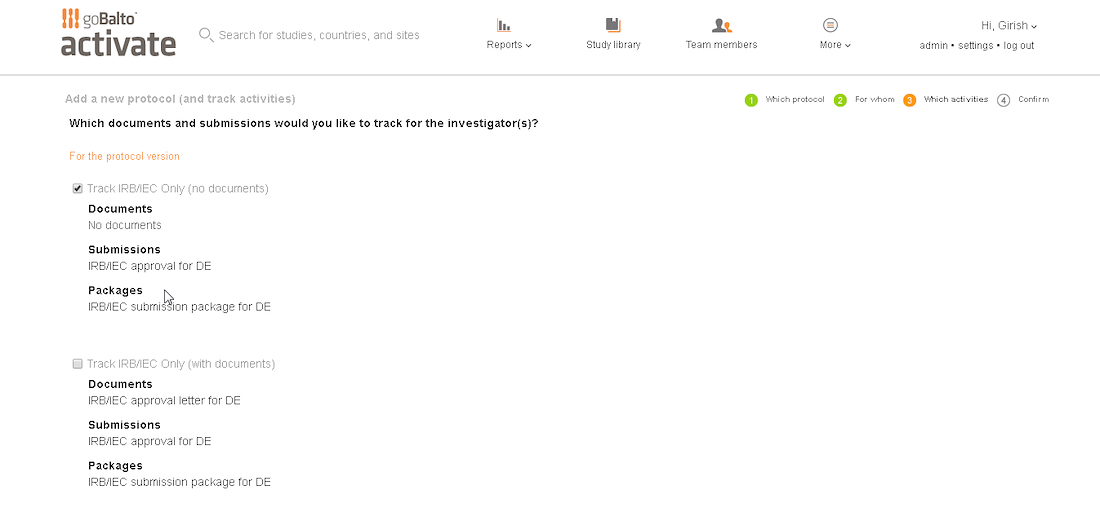
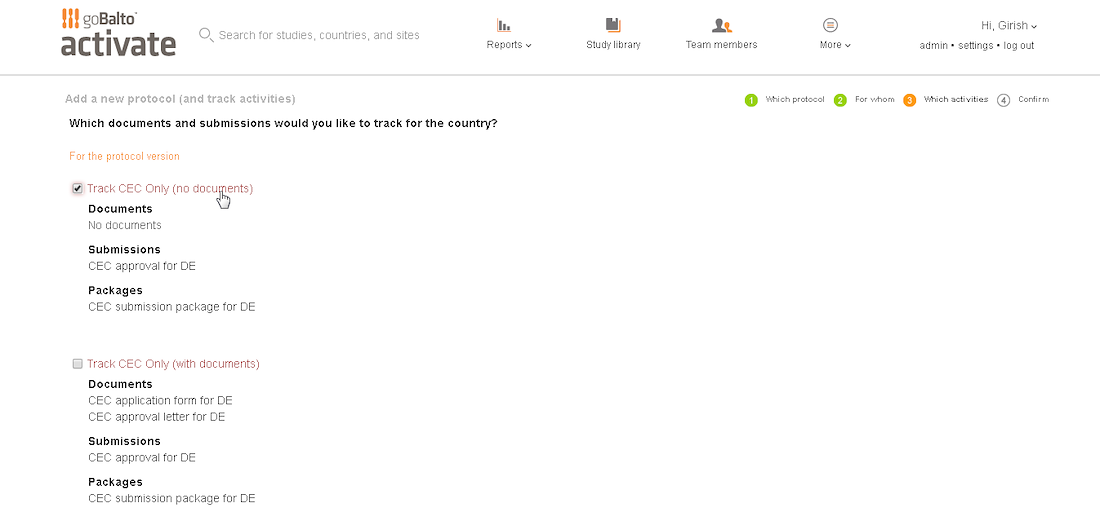
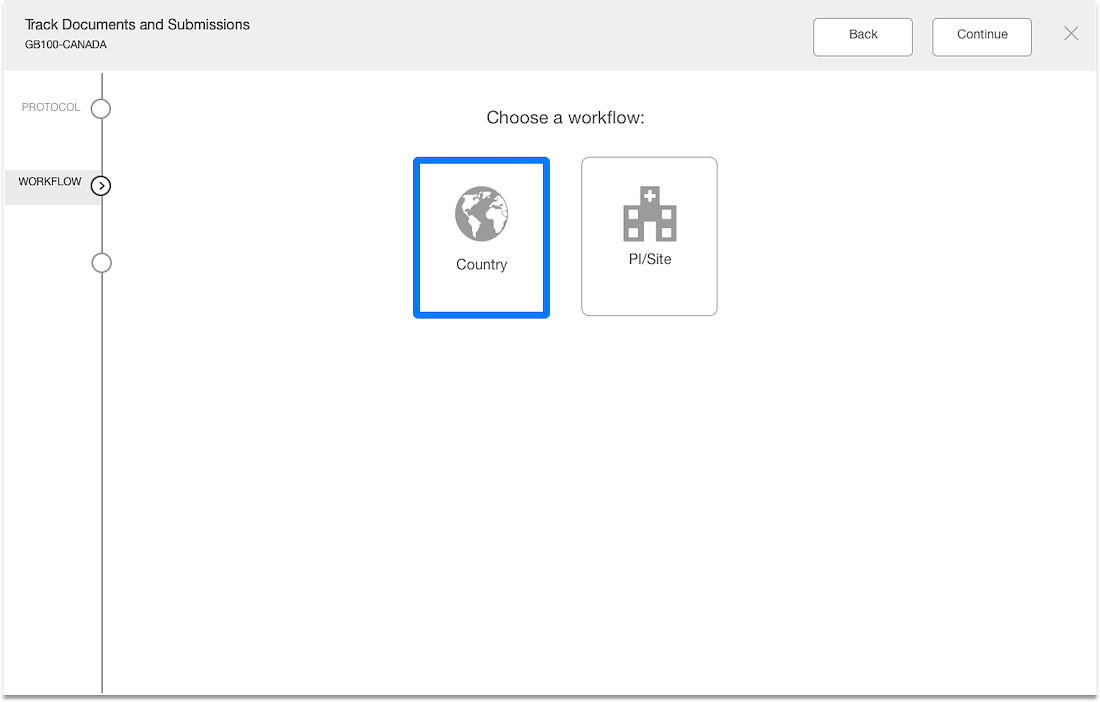
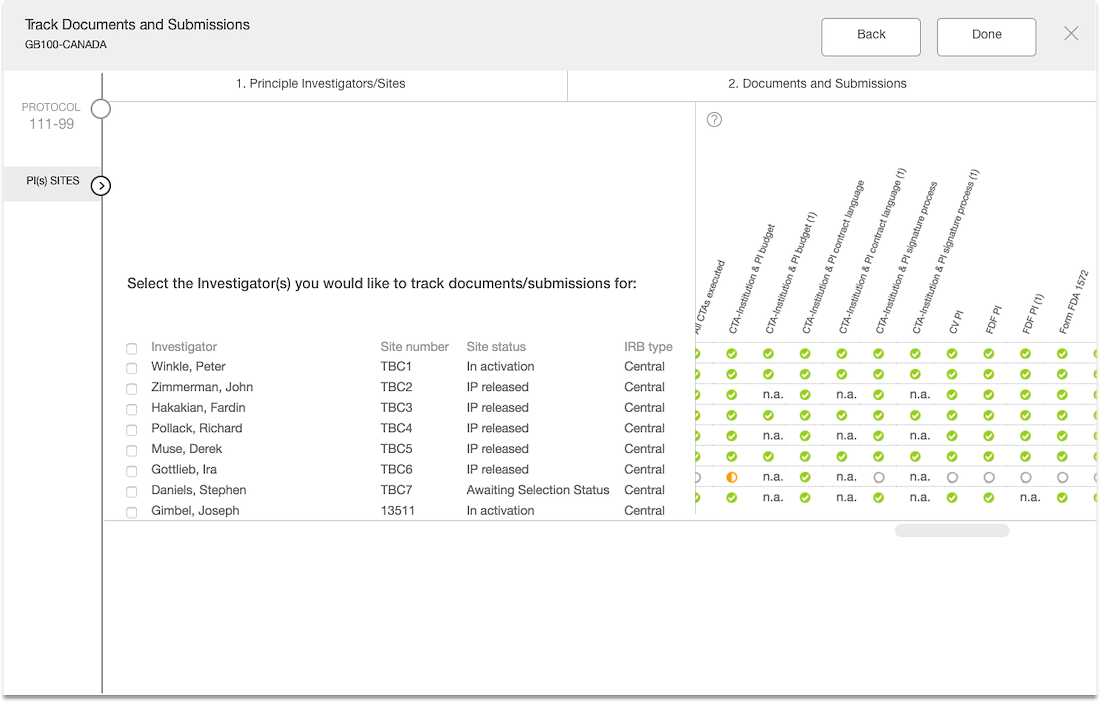
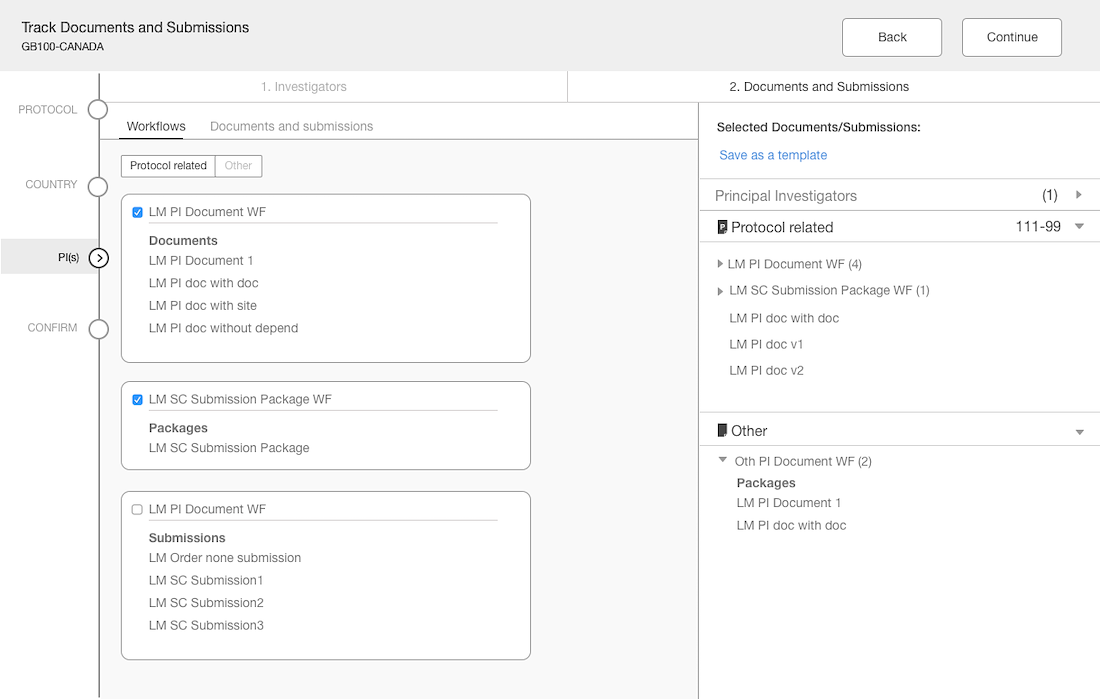
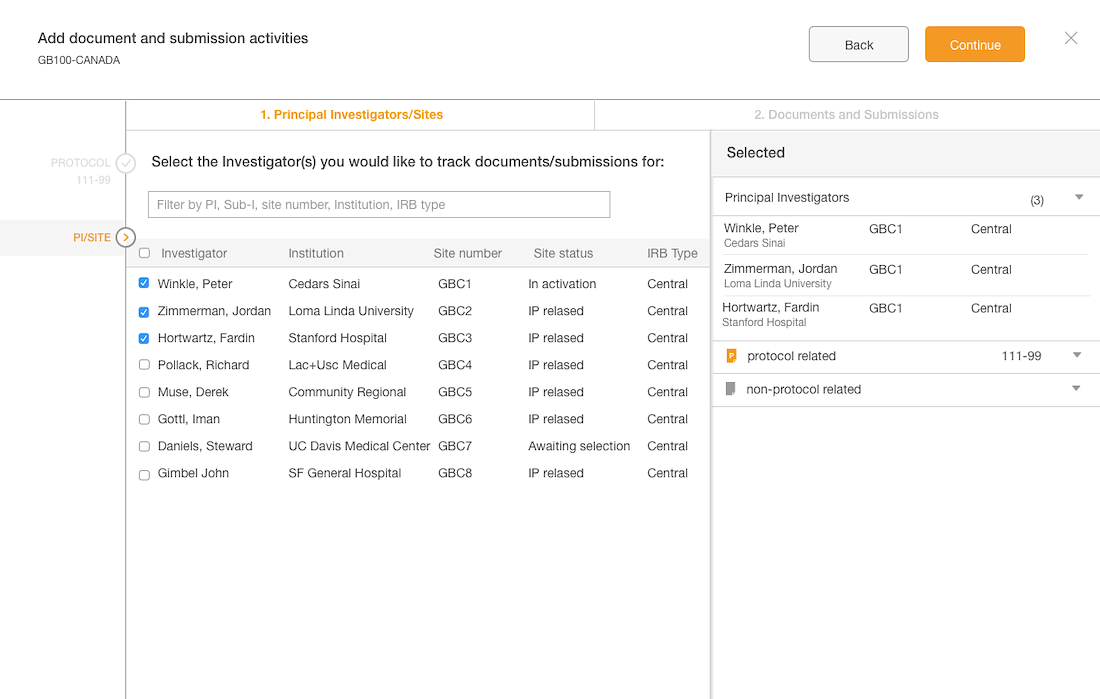
Workflow Wizard Legacy UI
This section dissects the UI and highlights the areas that need improvement
Findings
01 /
Set up process
Collections for Country vs PI/Sites have a different set up process that should be separated
02 /
Human error
- Users often create duplicate workflows because it is hard to keep track of documents added
03 /
UX
- The step by step flow could be improved
04 /
UI enhancements
Improving visual hierarchy/style & components can help organize the content & data
Problem
- The steps it takes to complete a workflow are visible, which is helpful for users.
- It is unclear what level the user is creating a workflow for since the Study or Country are not visible in the UI.
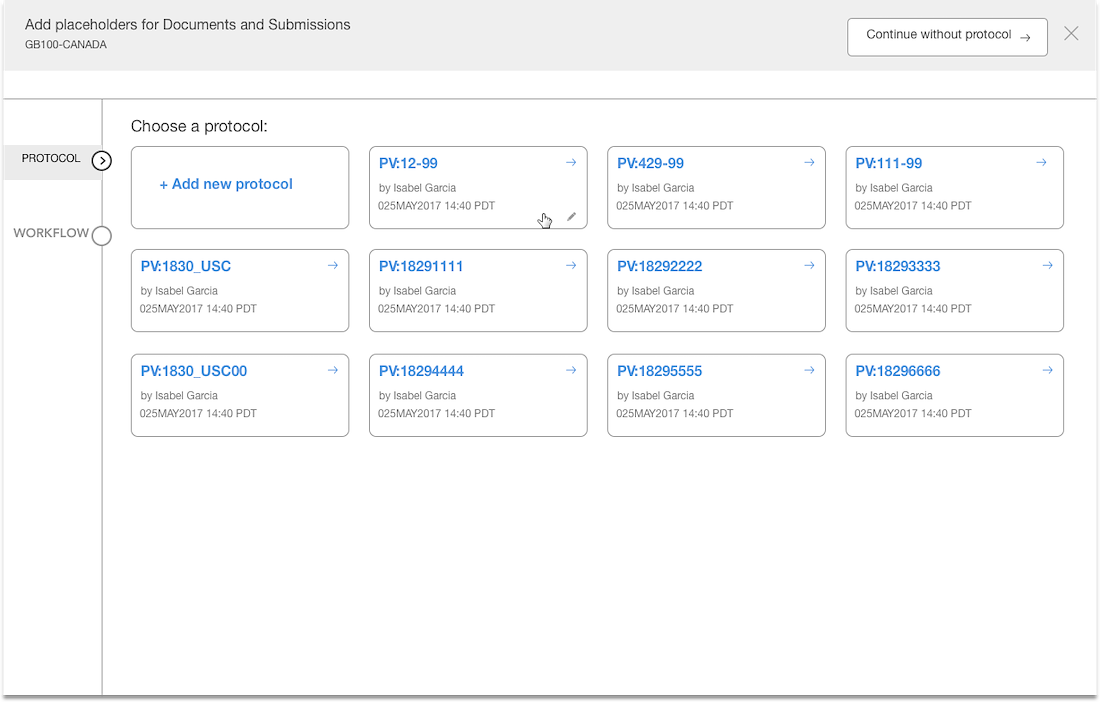
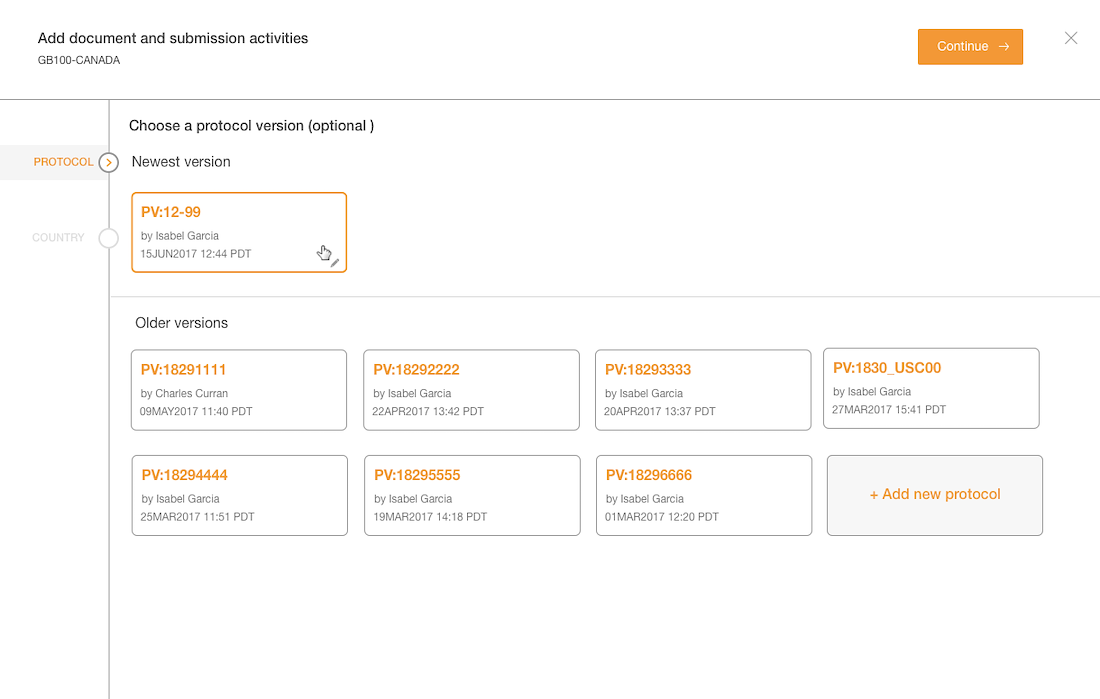
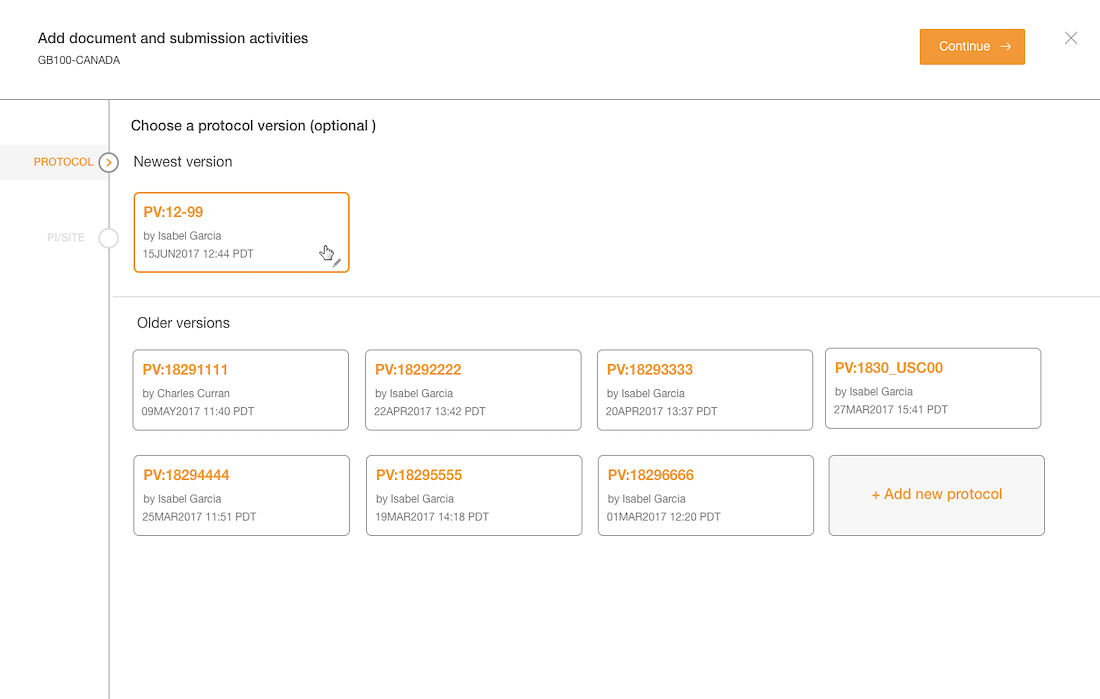
- Protocol versions are not a required step and the UI doesn't communicate well. The
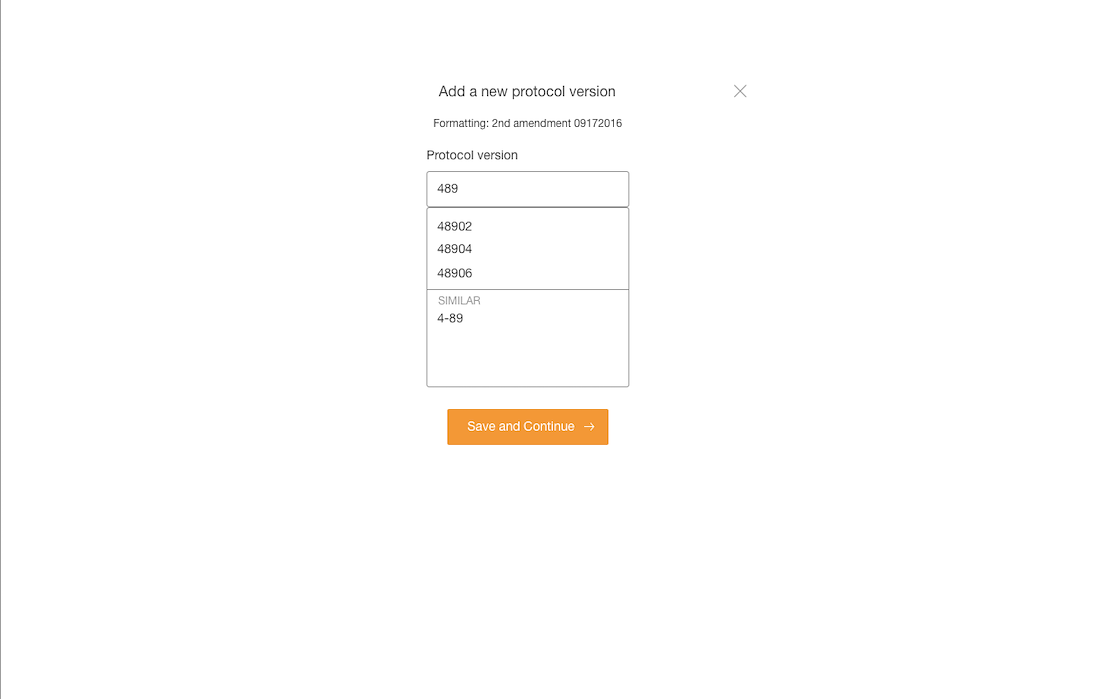
- Interaction for finding protocol versions that exists or adding new ones was confusing to users.
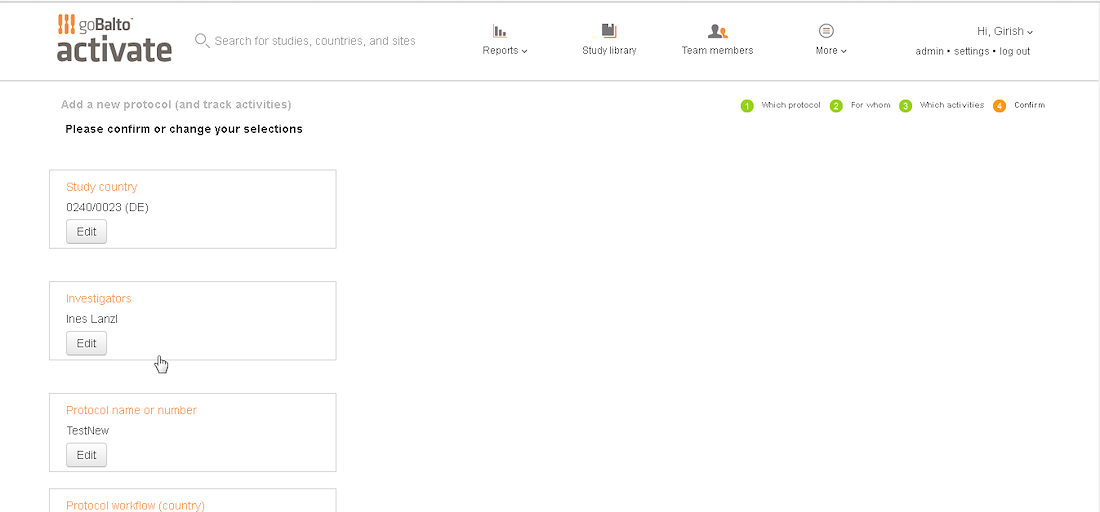
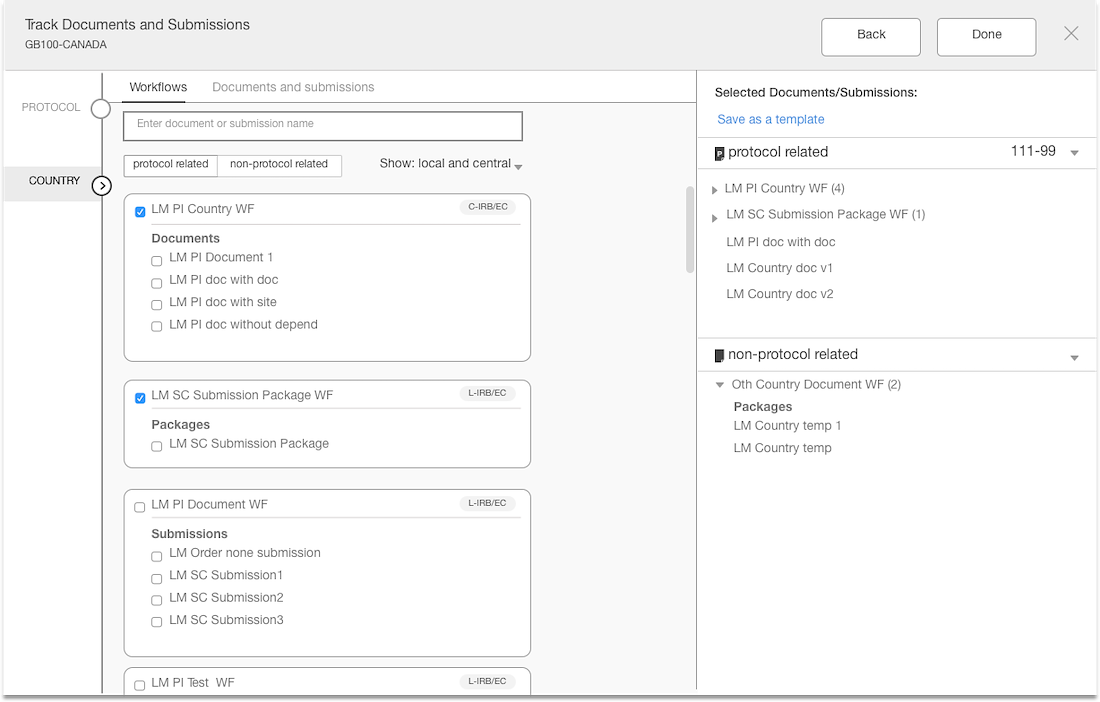
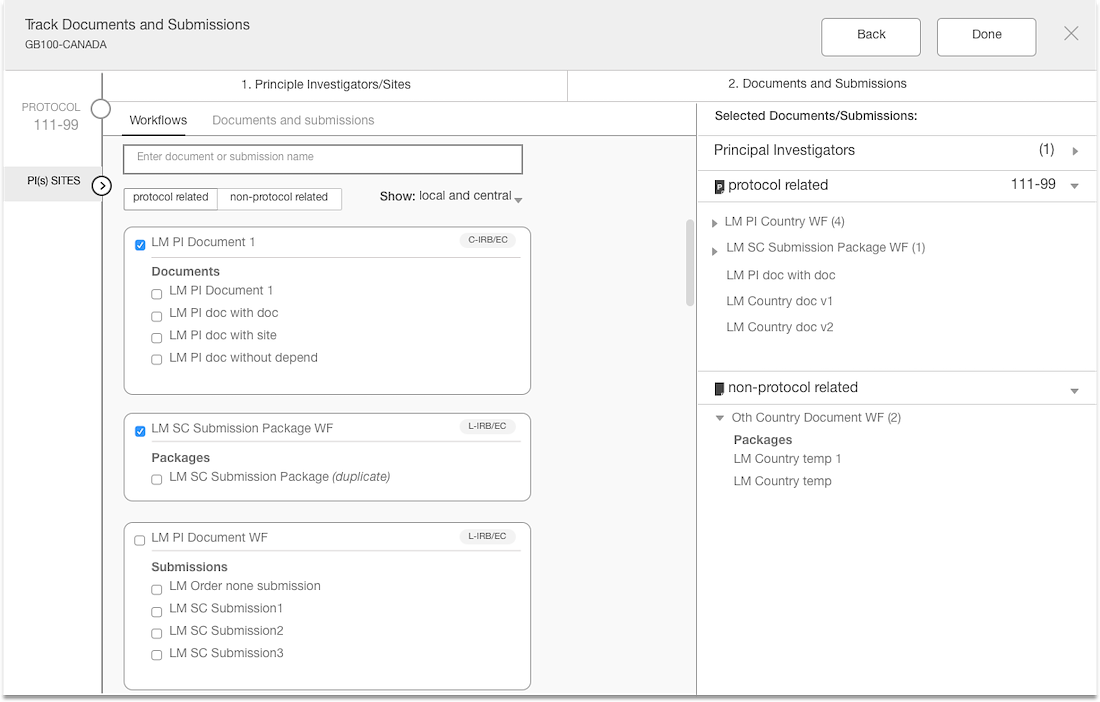
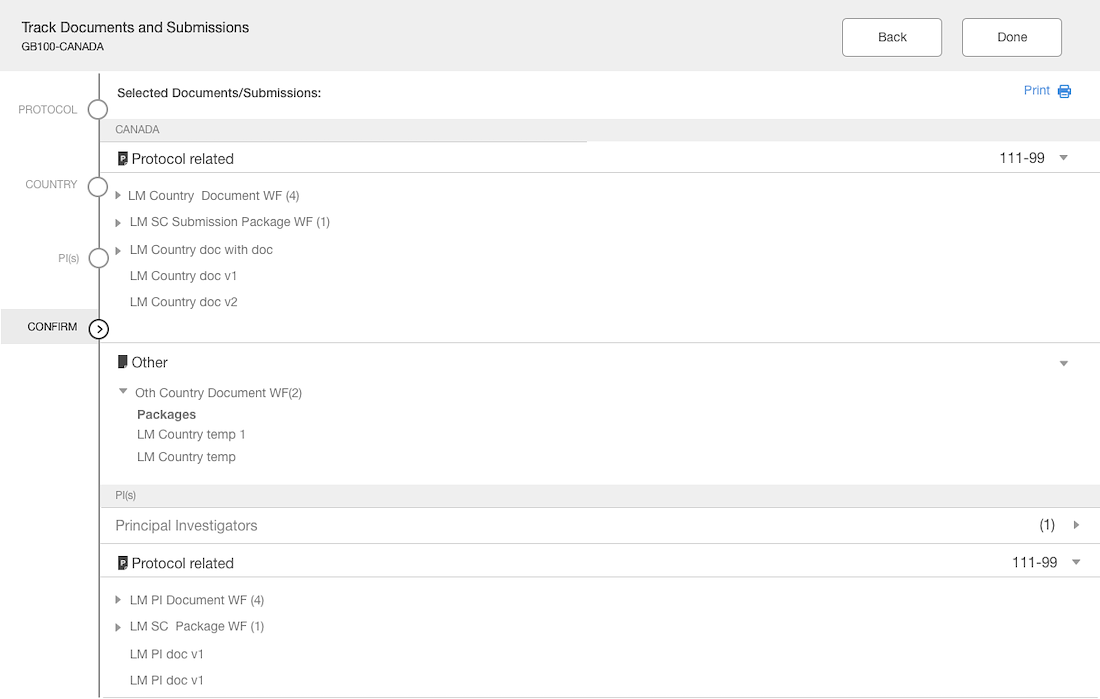
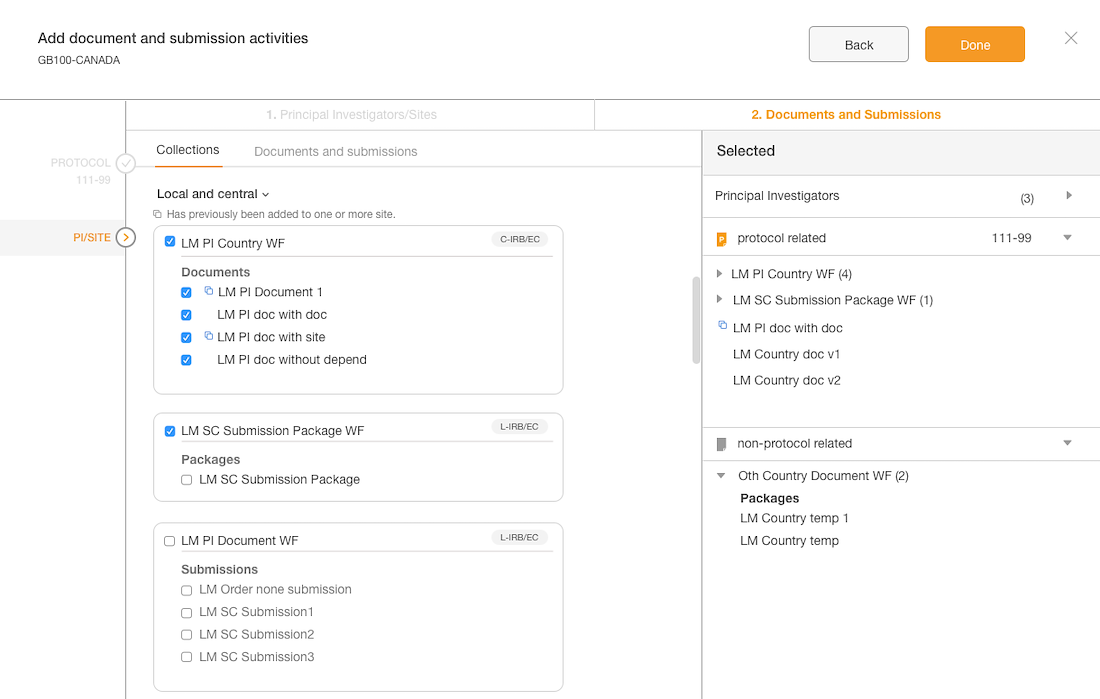
The Outcome
The interface is simple with unnecessary element
The layout is purposeful and the actions are strategically placed
Best practices of hierarchy are used to make for intuitive user experience
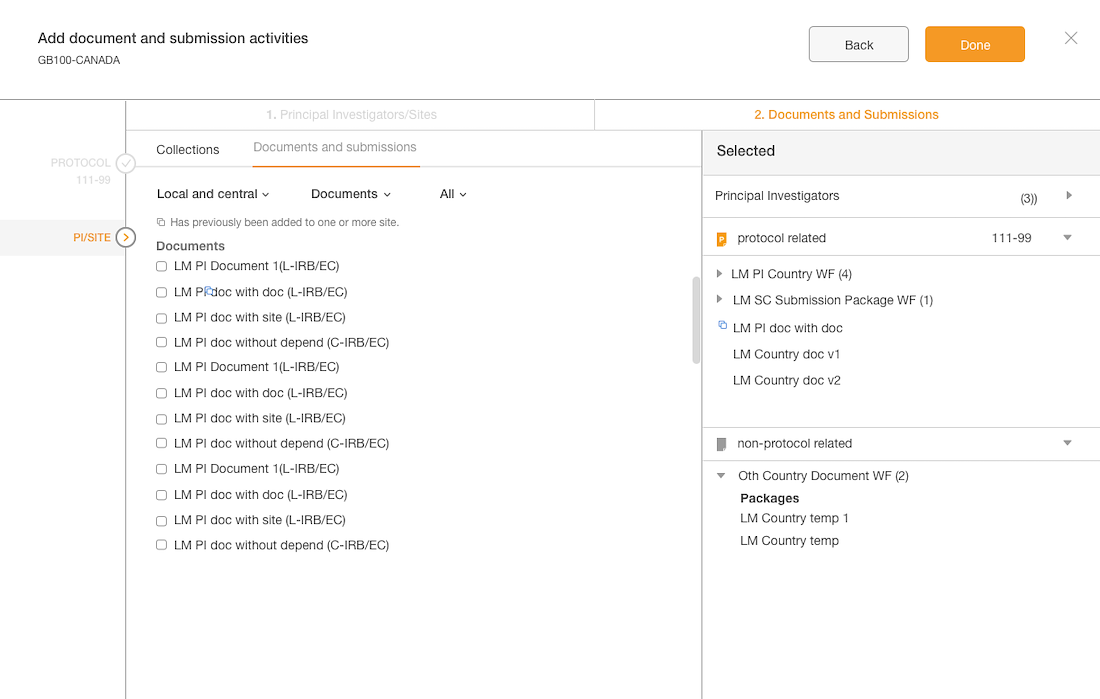
Introduce an intuitive solution
Introduced a flow with clearer steps
Validated the language with users
Enhanced the styling and components

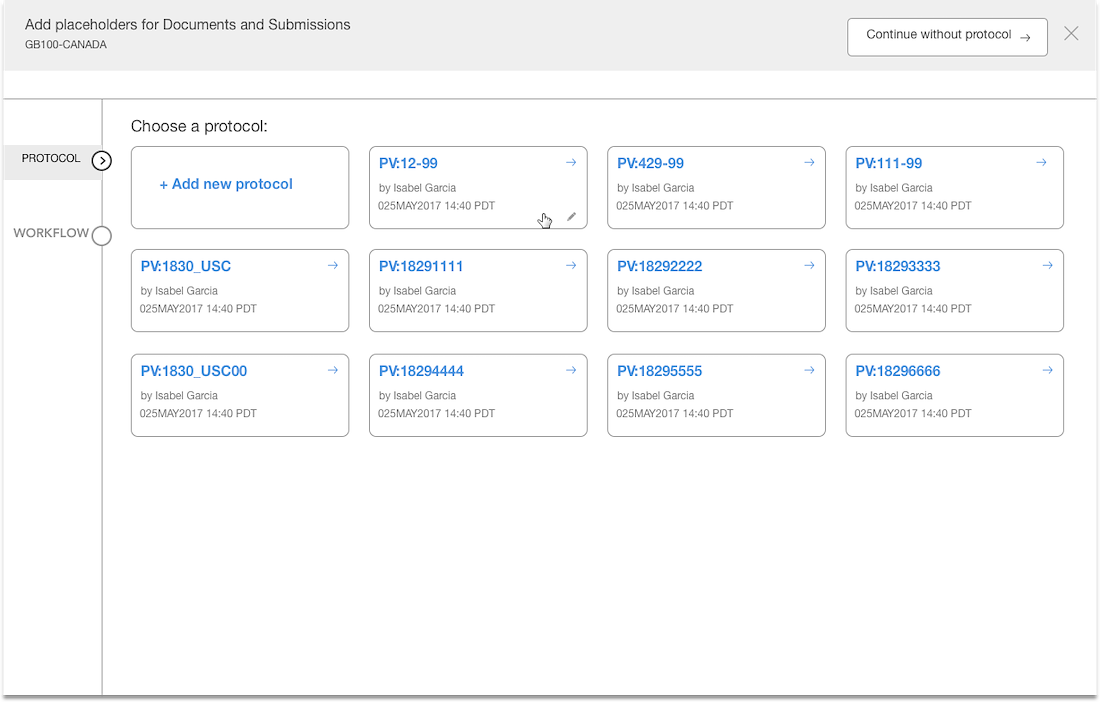
Reducing human error..
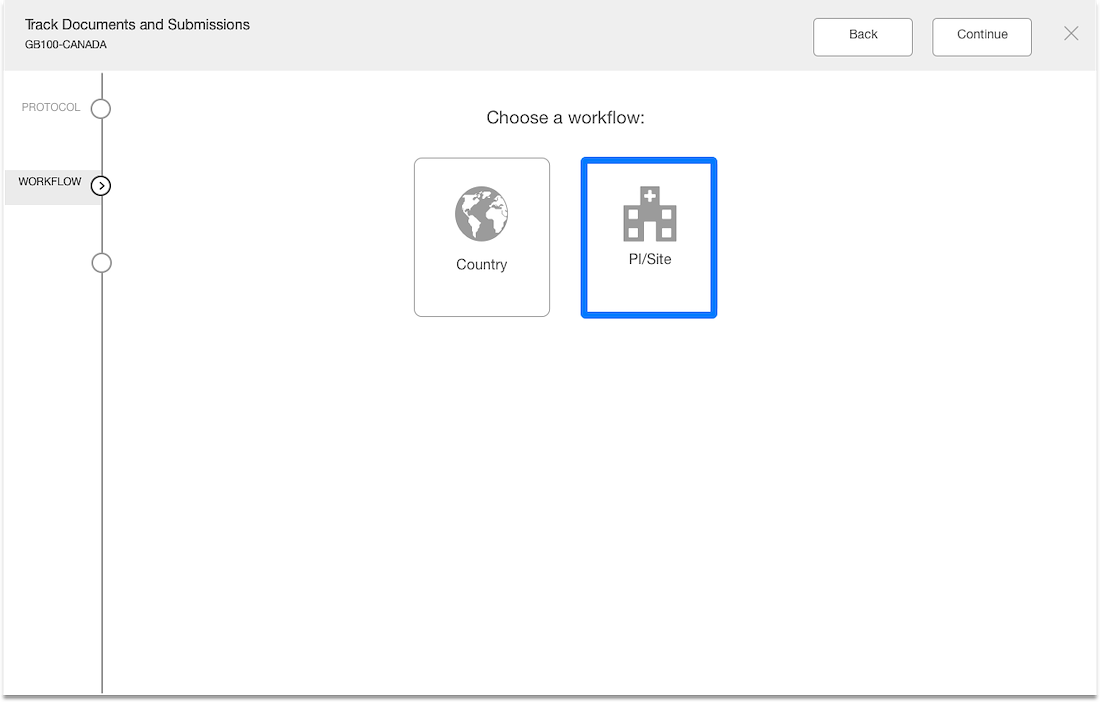
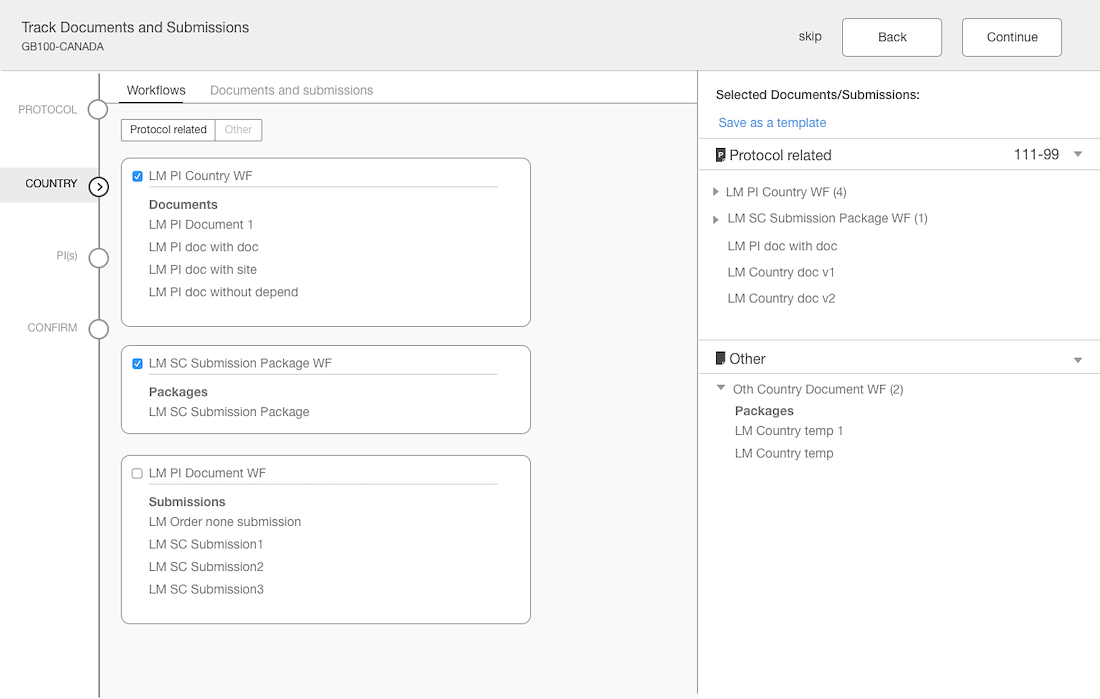
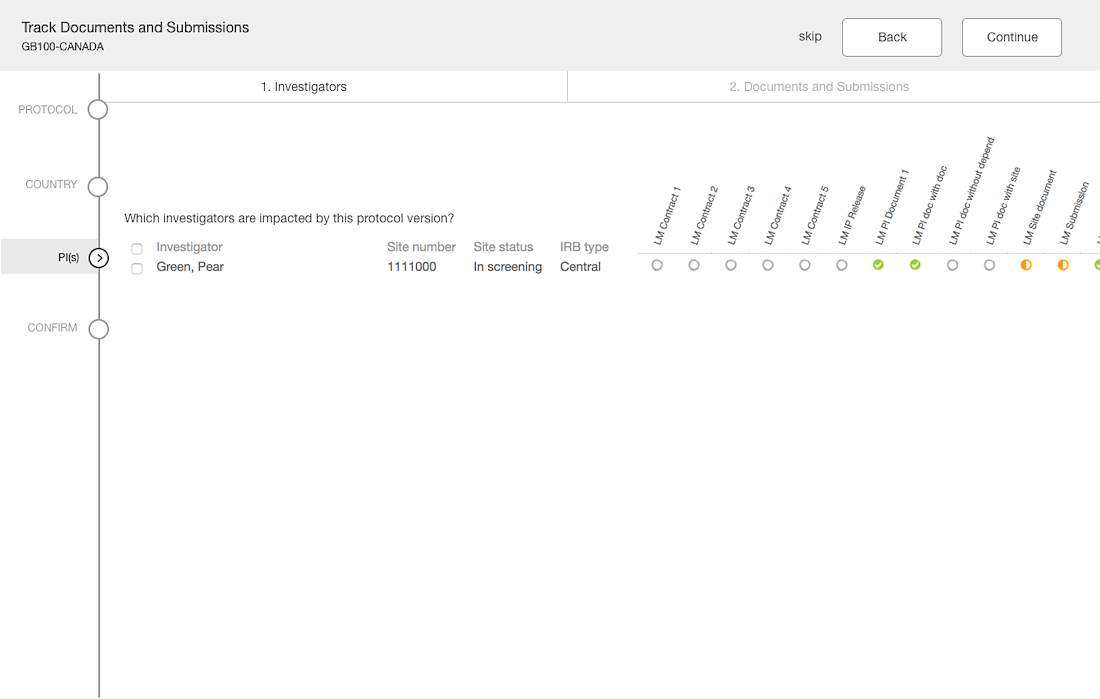
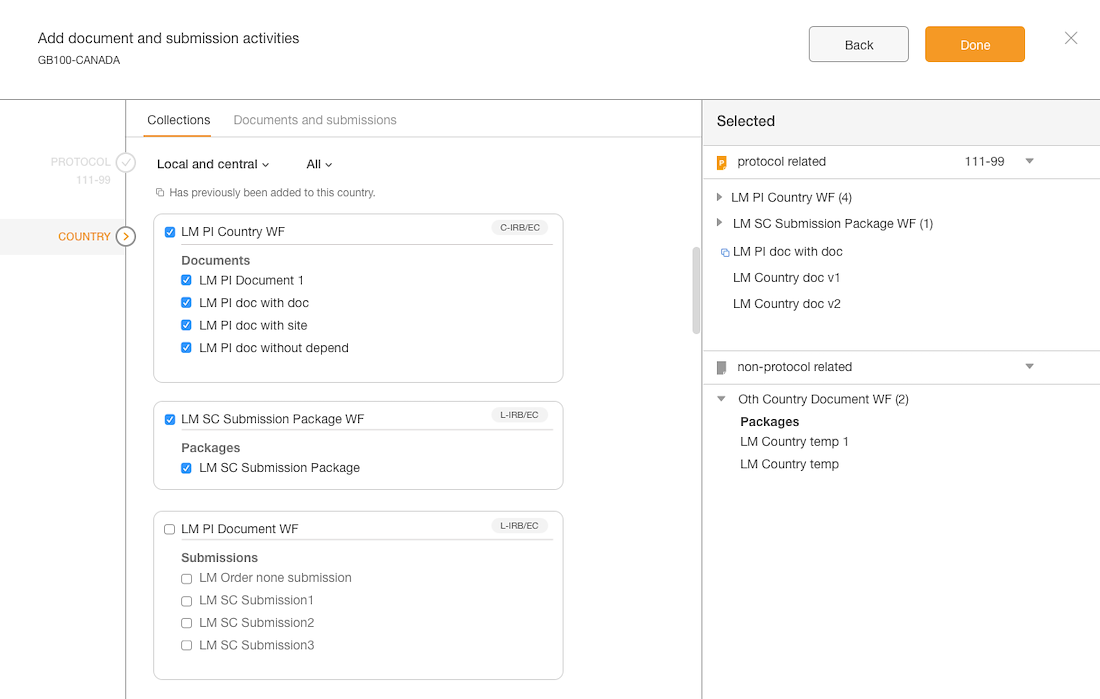
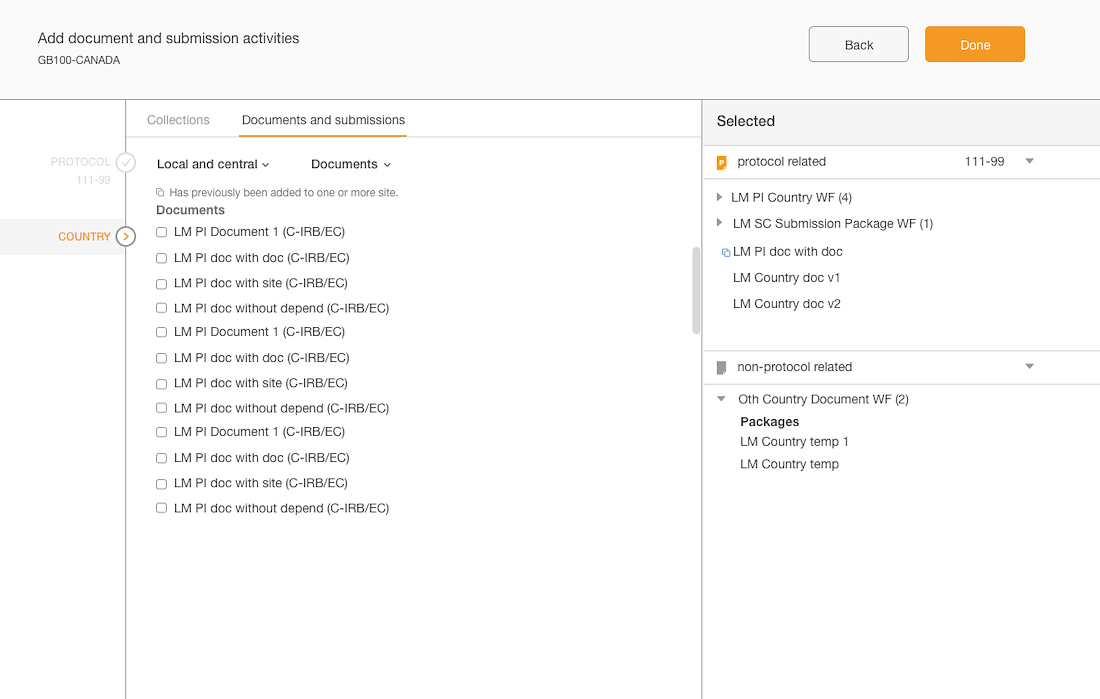
Separation of documents based on country & site levels
Added panel “shopping cart” that displays what documents have been added
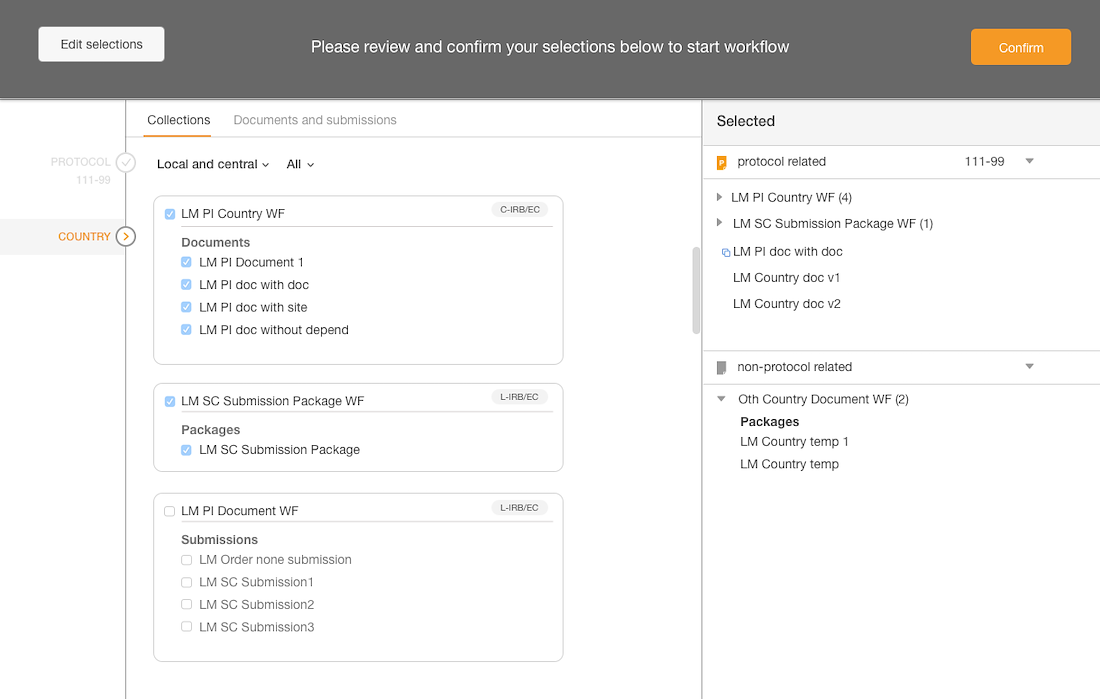
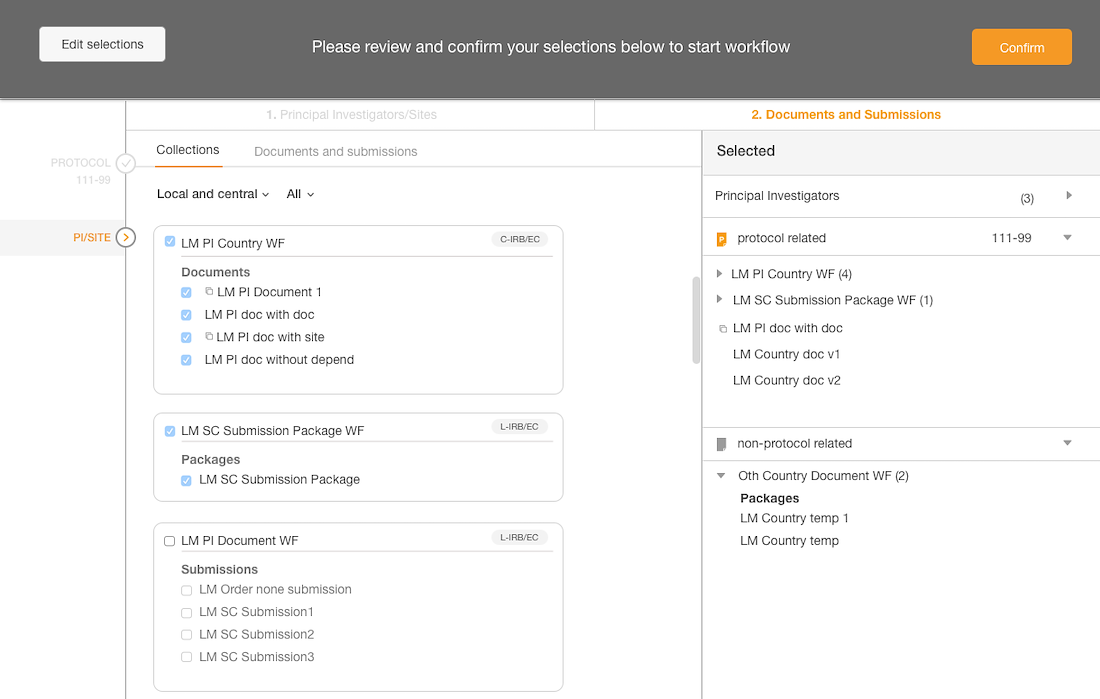
Introduced a confirmation step with the opportunity to edit

Final Thoughts
- • Taking the time to use my internal resources guided me to find a solution
- • Understanding users needs was important and validated the final design
- • Our data shows that the number of mistakes and tickets associated with human error have reduced since the new design has been implemented
- • The new component has been re-used for other areas in the product that handle documents
- • The biggest challenge was lack of knowledge in the Life Science space and it was difficult to do competitor research for this feature